
You are:
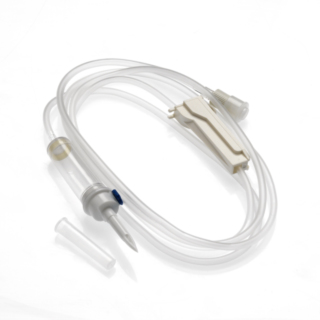
The INFINEED sterile, DEHP free, BPA free 1-way infusion device has been designed to minimise its environmental impact, using only raw materials that are free from endocrine disruptors (elimination of components, including DEHP or BPA). Infuser with: Length: 300 cm Two-channel perforator with grip wings. Air intake: Delivered closed for immediate use on a 0.2µ filter bag. Chamber: Flexible and transparent, Calibrated at 20 drops = 1ml ± 0.1ml, 15µ particle filter. Flow regulator: Accurate and reliable, linear flow regulator. Tubing: PVC (DINCH or TOTM) designed for comfort, optimal flow adjustment consistency and to avoid kinking. End fitting: Mobile Luer Lock. End stopper: Equipped with a purge filter with anti-bacterial membrane.
Residual volume: 230 ml
Indications
This device enables the parenteral administration by gravity of injectable preparations: parenteral and photosensitive drugs, solutions and nutrition when long tubing is required.
For peripheral lines, the infusion line can remain in place for a maximum of 96 hours (i.e. the maximum time the peripheral catheter can be in place).
For central lines, the infusion line must be changed at least every 72 hours (or according to the institution's validated protocol).
Labeling
on each unit are written the reference, the lot/serial number, the sterilization method and the expiry date if applicable
medical device
sterile
conformity
economic operator
Transport conditions
Protected from light, humidity and heat
| Conditioning | Parts quantities | Dimensions in cm | Weight in kg | Ean 13 of conditioning |
| UNIT | 1 | 0.040 | 3661809032738 | |
| BOX | 50 | 24 x 39 x 29 | 3.740 | 3661809032745 |
| CASE | 200 | 42 x 50 x 40 | 9.620 | 3661809032752 |